Preface
Healthcare worldwide currently faces both threats and opportunities. Digitalisation, new technologies and better use of health data point to a bright future. Healthcare in the future will be able to address, in a personalised and preventative way, diseases that we previously thought beyond hope. At the same time, we see the cost of treatment rising to levels that society cannot afford. The picture is bleak, due to overstretched healthcare systems and a shortage of healthcare professionals. These threats can distract us from the opportunities that lie ahead.
Even when we see the potential of health data and artificial intelligence and want to use them to improve public health or research and innovation, we often end up spending a lot of time and resources on lengthy administrative permit processes. Sometimes the data that is needed is hard to find, and when we finally get access to the data we need from multiple sources, it is not comparable. In the worst cases, it cannot be used.
At the start of the current European Commission in 2019, Commissioner for Health and Food Safety Stella Kyriakides received a mission letter from Commission President Ursula von der Leyen who stated that the Health Commissioner should work for:
“The creation of a European Health Data Space to promote health-data exchange and support research on new preventive strategies, as well as on treatments, medicines, medical devices, and outcomes.”
The mandate to improve the use of health data therefore came from the highest EU (European Union) policy level. Preparations for the TEHDAS project started shortly after the letter, and the kick-off between organisations from 25 countries took place in early 2021. For 30 months we worked to help Members States and the Commission develop and promote policies necessary for sharing of data in secondary use for purposes of citizens’ health, public health, and health research and innovation in Europe. The mission of this joint action has had several dimensions, including sustainability and outreach, governance, data quality, infrastructure, and the role of citizens.
In May 2022, the European Commission issued the proposal for regulation for the EHDS. The impact of the TEHDAS project can be seen in the proposal.
This report presents the main results of the project. For more detailed results I urge you to read the final documents that are available on the tehdas.eu website.
I would like to thank everyone involved in this project, including all partners, stakeholders, the European Commission and external advisors, but especially the team at the Finnish Innovation Fund Sitra, who worked hard with me starting from a blank page to a successful implementation of TEHDAS.
It will be possible to provide personalised and high-quality health services, and this can be achieved in an economically sustainable way, but only if we use data effectively and securely.
14 September 2023
Markus Kalliola
TEHDAS Coordinator
Project Director, Health Data 2030, The Finnish Innovation Fund Sitra
Summary
The joint action TEHDAS (Towards the European Health Data Space) supported Member States and the European Commission in developing the secondary use of health data in the EU.
Member States are better prepared due to the active dialogue and engagement of key stakeholders as part of the project’s outreach activities. Twelve Member States were visited to better understand their views and expectations on the EHDS (European Health Data Space). All countries recognised the benefits of the legal proposal, but some practical and legal challenges were still identified that need to be resolved for the successful implementation of the EHDS.
The results also highlighted the need for a trustworthy governance framework to ensure that health data can be shared for research and policy purposes while maintaining public trust. An analysis of problems and proposed solutions was conducted to establish trustworthy governance framework to boost people’s trust in data sharing for secondary purposes, and to enable the establishment of a system where data sharing is responsible and safe.
The main barriers to the implementation of the EHDS are related to legal aspects, pointing to the need for harmonisation and guidance on the application of the GDPR (General Data Protection Regulation). Efforts in this area have also resulted in concrete cooperation between Member States and advanced recommendations for the organisation of health data management in the EHDS.
Data quality efforts included an analysis of existing interoperability standards and initiatives, and collaboration with European Medicines Agency to promote alignment with the Data Quality Frameworks proposed by both actors. The main outcome was a data quality framework outlining the key components to enable semantic interoperability.
Cross-border collaboration requires also technical interoperability of infrastructures. A conceptual model of a pan-European distributed infrastructure for the secondary use of health data was presented, followed by a user journey illustrating concrete steps a data user needs to take to access data.
Security is always a key factor in the use of health data. An analysis of secure processing environments provided a fertile basis for discussion on how they could be implemented in Europe in the future.
Public engagement aimed to better understand people’s attitudes towards sharing their health data. The work also included raising awareness of the benefits of the secondary use of data for individuals and patients. The results showed that people are interested in sharing their health data, but with clear safeguards for privacy and security. Furthermore, the work on data altruism, where people voluntarily share their data for the common good, has led to guidance on implementation mechanisms that respect data protection regulations.
Overall, the project has provided a solid basis for ensuring the relevance and fit-for-purpose legislative development in the area. It has promoted access to health data across borders, with the aim of stimulating research, innovation and policymaking for better public health, ultimately leading to better health for all.
This project has been co-funded by the European Union’s 3rd Health Programme (2014–2020) under Grant Agreement no 101035467.
TEHDAS in numbers
1. Unlocking health data for everyone’s benefit
The EHDS (European Health Data Space) will improve the cross-border use of health data in the EU for the benefit of individuals, policymakers, business and research.
Secondary use of health data and the EHDS
The EHDS is a proposal for a regulation that will support individuals to take control of their own health data and promote the use of health data for better healthcare, as well as better research, innovation and policy making. The EHDS will play a key role in facilitating health information exchange, data sharing and secondary use of health data across the Member States.
It is worth noting that the EHDS is not a centralised repository of data. Rather, it can be characterised as a data sharing framework with harmonised and clear rules for data access and use. Overall, the aim of the EHDS is to establish governing principles, rules and infrastructures for data sharing that will enable large-scale collaboration across Europe. Such, it also provides a vision for the future of health and healthcare in Europe.
The use of health data can be divided into two main categories: the primary and secondary use of health data. When a patient is diagnosed with a disease, receives treatment and prescribed medication, data is generated and stored. This is the primary use of data. The secondary use of health data means that the data generated during for example healthcare or clinical trials is used for other purposes than for which they were originally collected. For example, when data on many patients with the same medical condition and the same or different treatment is compared to examine the effectiveness of the treatment, this is considered secondary use.
The joint action TEHDAS (Towards the European Health Data Space) has focused on the secondary use of health data. Therefore, its focus is narrower than the whole EHDS, which also covers the primary use of health data.
TEHDAS joint action
The TEHDAS joint action supported EU Member States and the European Commission in building a European Health Data Space by developing principles for the cross-border secondary use of health data. Joint actions are a funding instrument under the EU Health Programme. They are designed and financed by Member State authorities and the EU to address specific health priorities.
The joint action has provided a forum for partners and stakeholders from different Member States, other participating countries and the Commission to collaborate on policy, legal, governance and organisational issues related to the secondary use of health data. By also addressing data quality, technical and interoperability aspects, TEHDAS has addressed several elements that are crucial for the development of the EHDS and the promotion of the secondary use of health data.
TEHDAS was carried out by 25 European countries and co-ordinated by the Finnish Innovation Fund Sitra. The project started in February 2021 and ran until July 2023. It was based on the European Commission’s Health Programme 2020 and co-funded by the EU and the participating countries.
2. Results: Mobilising health data in Europe for research, innovation and policymaking
The project successfully produced recommendations to create, further improve and implement effective legislation on the use of health data in Europe. Its influence is well reflected in the Commission’s legislative proposal on the EHDS and will support the implementation of the regulation.
Towards EHDS through dialogue and collaboration
Building networks, raising awareness and facilitating dialogue between different stakeholders are key to building a successful EHDS. Currently, Member States vary widely in the way they organise and manage health data as well as in their readiness to benefit from the EHDS regulation.
Several projects funded by the European Union have promoted and developed the use of health data prior to the TEHDAS project, and many projects were running at the same time. EU Member States have also used health data in very different ways. It is important to involve all the relevant stakeholders in the process of setting up the EHDS. In order to learn from others and engage all stakeholders in a dialogue about the EHDS, TEHDAS organised Project Forums, Policy Forums and fact-finding country visits.
The country visits involved interviews with local stakeholders who work with or exchange health data, such as researchers, healthcare providers, registries, insurance companies, ministries of health and research, public health institutes, data protection authorities and digital health governance officials. This provided an overview of how health data is managed in different countries, and their needs and expectations for the EHDS concerning organisation, technical, legal and resource aspects.
The results of the country visits are summarised in publicly-available factsheets. The factsheets provide a snapshot of the state of play of the health data management system in 12 countries across Europe, namely Belgium, Czech Republic, Denmark, Estonia, Finland, Germany, Hungary, Ireland, the Netherlands, Portugal, Slovenia and Sweden. They outline the needs and expectations of stakeholders that have to be taken into account when setting up the EHDS, and reflect on the readiness of different countries to join the EHDS.
Overall, all countries saw the benefits of the EHDS and had the political will to join it. These benefits include, for example, the facilitation of research studies that require access to health-related datasets and the use of common European templates and guidelines in the process of reusing health data. However, there are still many practical and legal challenges to overcome, such as the fact that there are different data management systems in different countries. Stakeholders also raised concerns about the timing of the implementation of the EHDS regulation, indicating the need for more time and resources, including human, technical and financial resources.
The country visits and the associated mapping of health data management in different Member States and associated countries contribute to the development of a sustainable and pragmatic EHDS, ensuring that it is based on a realistic state of development of health data management and sharing across Europe.
The country visits also provided an opportunity to reflect on needs and expectations at national level and on how TEHDAS and the EHDS could respond to them. Importantly, these efforts also provided an opportunity to bring together stakeholders involved in the health information systems, and get an overview of the current situation in their country regarding health data management and the regulations supporting the exchange of health data for secondary use.
As part of the outreach activities, two types of forums were organised to discuss the EHDS both at 1) technical level (Project Forum), involving other EU projects and initiatives working on the secondary use of health data, and 2) political level (Policy Forum), involving representatives from the ministries of health, research and finance.
The Project Forum helped create co-operation, synergies and facilitate learning between EU initiatives and projects that work on the secondary use of health data. The Policy Forums, on the other hand, provided a sounding board for European ministries to learn about the developments in the EHDS and voice their needs, concerns and expectations for the EHDS in a unique environment where different ministries from different countries were all involved in the same conversation.
Policy makers were initially concerned about the legislative proposal for the EHDS and how it would fit into the current legislative ecosystem. They were also interested in learning more about the EU funding available for the implementation of this legislation and the HealthData@EU pilot project. Importantly, the Policy Forums brought together representatives from three different ministries – health, economy and research – because they will all be affected by the EHDS. For example, representatives from ministries of finance were very much involved in the discussion on the sustainability of the EHDS for secondary use and the introduction of a fee policy to maintain the infrastructure.
Figure 1. Twelve countries participated in the TEHDAS country visits.
Both EU and national legislation need to be clarified
At present, health data is managed and administered in very different ways across the EU, which hinders the use of data. To accelerate the use of health data, sustainable governance options for the EHDS and guidelines for national legislation on data sharing are needed.
Different national governance systems, lack of standardisation of data sets and variations in legal interpretations of EU data protection law are examples of the most common barriers that make transnational studies difficult and increase the costs of research and compliance.
Other examples of barriers include differences in data access procedures and lack of harmonised definitions of key terminology.
During the project, a Memorandum of Understanding (MoU) was signed between Findata (the Finnish Social and Health Data Permit Authority) and the French Health Data Hub. Although a success, the MoU did not cover the cross-border exchange of health data, a clear indication of the legal complexity of such activities. However, these efforts were very fruitful in providing a best practice and a concrete template for a MoU or similar agreement for cross-border collaboration between health data-related institutions.
TEHDAS published proposals to overcome the identified barriers to data sharing. This document identifies several policy options for each barrier, ranging from proposals on improving the clarity of EU data protection law to proposals for improving data quality and interoperability (Table 1).
Table 1. Barriers to cross-border sharing of health data for the secondary use are often related legal issues, but also data, transparency and trust.
| Barrier description | Theme |
| There are differences in governance and health data systems in Europe. | Infrastructure Legal |
| There is no common European interpretation of what constitutes ‘sufficient anonymisation’ to transform personal data to non-personal data. | Legal |
| There is no common European interpretation of what constitutes ‘pseudonymisation’. | Legal |
| There is no common European interpretation of what is, and what is not, ‘secondary use’ of data. | Legal |
| European countries have national legislation/rules concerning health and research data in addition to the GDPR. | Legal |
| European countries have the ability to set their own derogations under the GDPR. This lack of harmonisation may create additional barriers. | Legal |
| European countries have different preferences as to the choice of legal basis for processing under the GDPR. This impedes the cross-border collaboration and data sharing. | Legal |
| Health data is considered sensitive data, meaning for example special category data under GDPR, and is treated differently from other types of data when it comes to health data ethics, management and use. | Data |
| No standardised data sharing agreements exist for products developed by private sector providers using public sector health data to (a) facilitate safe data sharing and (b) protect taxpayers’ investment. | Trust and Transparency |
| Across Europe, different taxonomy and ontology codes are used to label the same health condition, making comparisons between data sets a challenge. | Data |
| Poor data management procedures reduce the ability to reuse data. | Data |
In addition, a guideline was produced to help researchers set up cross-border data-sharing partnerships, divided into six steps – from setting up partnerships to defining activities to promote the results of a partnership.
The current governance of the access to health data was studied in four countries: two with centralised systems (Finland and France) that provide a “one-stop shop”, facilitating health data access, and two with decentralised systems (Spain, and the Netherlands), where such an entity does not exist. Best practices and challenges in the functioning of these systems were described and a guide to setting up cross-border partnerships was developed.
After the presentation of the EHDS proposal, a forward-looking perspective was adopted. TEHDAS assessed how the access governance for projects using data from another country or from several countries would work based on the proposed EHDS regulation, and what issues still needed to be addressed in order to enable such projects effectively.
TEHDAS also suggested ways to clarify the proposed governance system from the European Commission’s proposal for an EHDS regulation, taking into account different forms of interoperability and ethical principles of trust. The analysis and compilation of different existing governance structures and mechanisms at EU level can be used for future development and revision of governance models, for the EHDS and other relevant regulations.
Data quality is essential for reliable and fit-for-purpose data
A data quality framework was created, which sets out the main elements of data quality and provides guidance to the European Commission and Member States on data quality and utility. The aim is to ensure that accurate, high-quality and useful data is available for secondary use while maintaining privacy and security.
Data quality is essential for research and innovation. Researchers and innovators need accurate data to develop a new drug, to find the causes of a disease, to understand which patients might benefit the most from a treatment, or to assess which prevention strategy is better to reduce mortality in the population. Likewise, policymakers and regulators need quality data, for example to safely introduce new treatments, to ensure that the most vulnerable patients receive the treatment they need, to assess gaps in the timely response to patients with critical acute care needs, or to tailor treatments to those patients that would benefit the most.
The TEHDAS data quality framework contains the main elements in data quality. These include the steps in the process of preparing data for research and innovation. Data collected in the course of providing care or as a part of a research project is not always immediately ready for further use. Often, additional preparation is needed to make the data findable and suitable for the secondary use purposes.
Data holders will use data quality assurance procedures to clean up the datasets and make them available providing researchers with a notion of the level of quality of the datasets they host. There are several activities that data holders will develop to ensure that data is of high-quality.
In addition to quality, data users also need data that is useful for their research purposes. Institutions providing access to data need to be aware that data may lose value when processed to enhance privacy and security. The quality framework presented also recommends to these institutions a balanced use of privacy-enhancing methods.
All activities aimed at improving the quality and usability of data are included as part of the extended user journey, the final stage of which will be the return of research outputs to data holders in a way that the data can be enriched.
Along the way, TEHDAS’ work has provided guidance to the European Commission and the Member States on a number of issues relevant to data quality and utility. For example, it has recommended the use of legal provisions to indicate the quality of a dataset, or the mechanism for informing data users about the value to them of a specific data collection. Likewise, the European Medicines Agency has used TEHDAS’ data quality work in its efforts to leverage routine data in the real-world evaluation of drugs and medical devices.
The impact of this work relies on the eventual adoption of the TEHDAS approach to data quality following the discussions on the EHDS legislative proposal; and, subsequently in the corresponding delegated and implementation acts.
It is time for the data quality and utility framework to become a reality. There are new initiatives in the European landscape that are pursuing the construction of HealthData@EU, a new decentralised EU infrastructure to support cross-border projects. Ensuring that these initiatives encompass the principles and recommendations of the TEHDAS data quality framework, as it currently appears, and providing the means for its implementation will have a significant long-term impact.
Ensuring secure data processing is crucial
The interoperability of technical infrastructures facilitates the efficient use of health data throughout the EU, while secure processing environments ensure a high level of personal data protection.
Providing secure and private access to health data requires the mutual interaction of many computational systems. For example, the information systems in hospitals or primary care services, the systems used by health authorities or ethics committees to grant access to the data or the secure processing environments used to process sensitive data. It is important that these interactions do not inadvertently expose patient data, and that once the data is provided to the data users, it is processed in a secure environment.
TEHDAS provided a comprehensive description of such systems, their interconnection and the intended interaction with all the human actors involved in this challenging scenario while ensuring personal integrity and privacy as well as security.
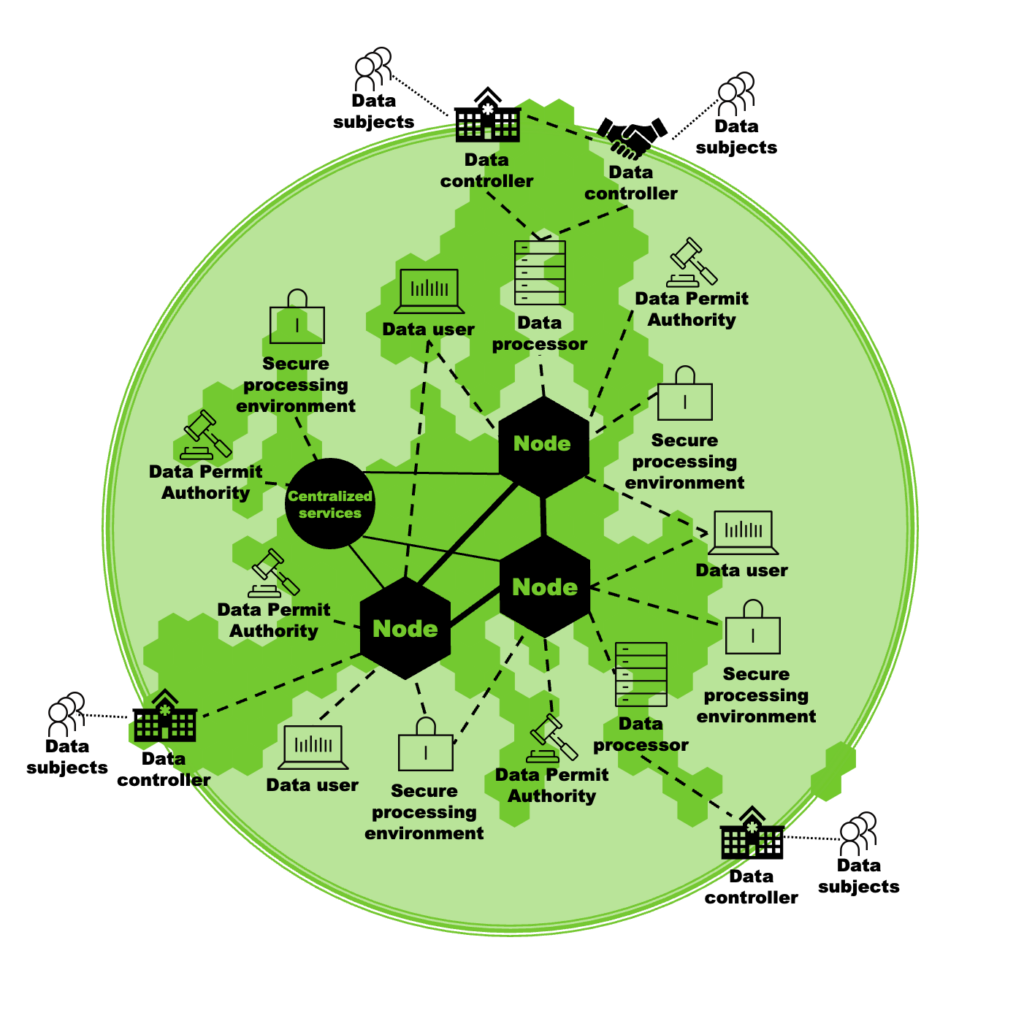
An architecture proposed by TEHDAS is shown in Figure 2 and foresees a network of nodes operated by the Member States that act as service providers for data users. These nodes connect the data holders and the data permit authorities that will decide on the granting of access to the data, as well as the secure processing environments where the data will later be analysed by the data user. The operation of the nodes will be supported by core services provided by European Commission.
Figure 2. TEHDAS’ proposal for architecture of the EHDS for the secondary use of health data
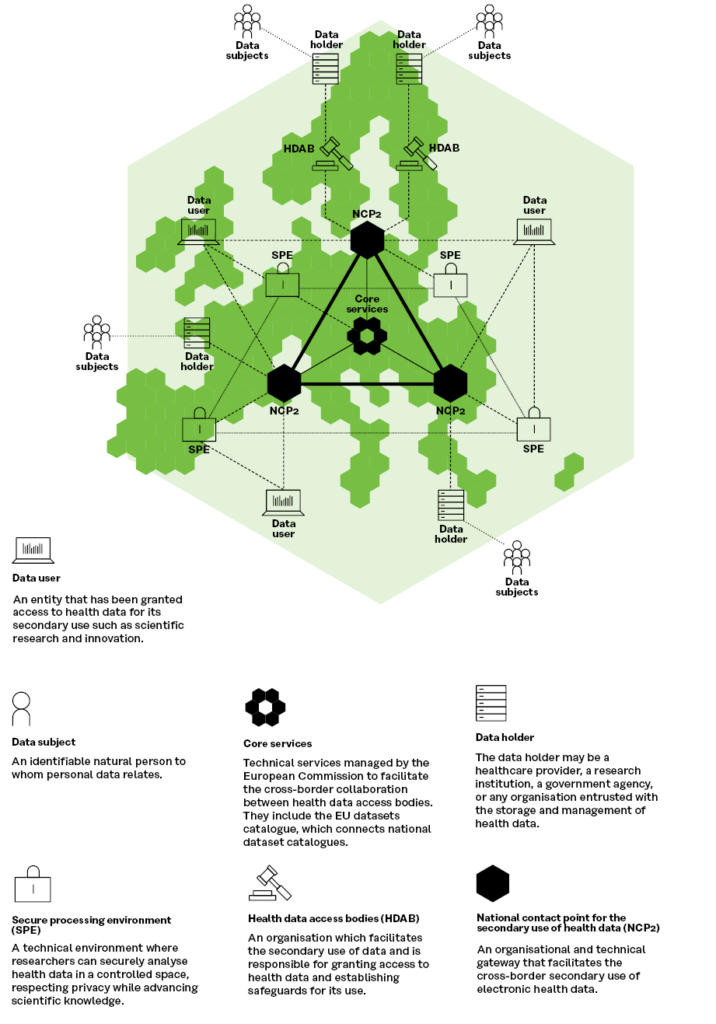
The overall technical architecture in the EHDS proposal and the TEHDAS architecture are almost completely identical, as can be seen in the Figure 3, where the differences mainly concern terminology.
Figure 3. TEHDAS’ proposal on HealthData@EU architecture for secondary use of health data (adaption of the EHDS legislative proposal)
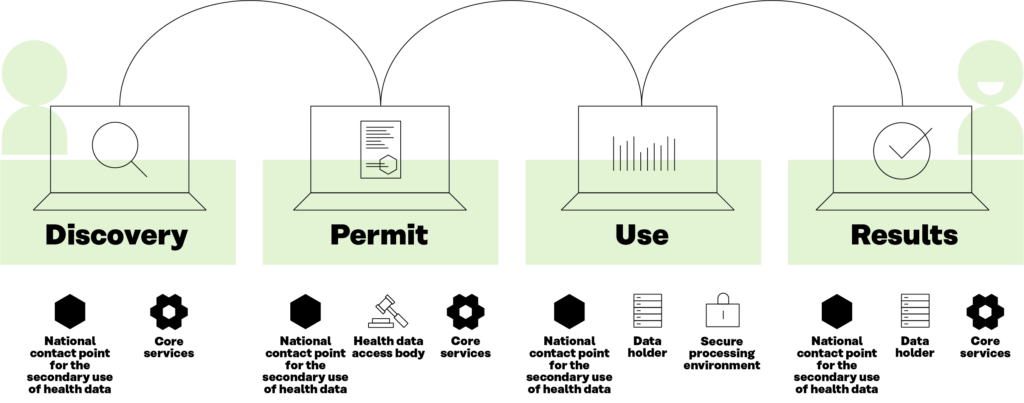
Another key outcome is the development of the Users’ Journey. This is a description of the process that the data user will use when interacting with the decentralised HealthData@EU infrastructure. This process is broken down into four main steps, as shown in Figure 4, related to the types of interaction with the data: 1) discovering the data; 2) applying for permits to use the data; 3) using the data effectively; and 4) publishing results. These building blocks will be the actual services to be implemented for the operation of the HealthData@EU.
The TEHDAS Users’ Journey has been adopted with minor modifications by the European Commission and is now serving as a guiding input for the development of the HealthData@EU pilot, a project where some of the technical solutions analysed in TEHDAS will be tested in a small group of Member States.
Figure 4. Data users’ journey in the European Health Data Space
Citizens support data sharing and use when the benefits are clear
People feel a special connection to their health data. They want to be involved in the process and have their values respected, with trust and transparency playing a key role.
A public consultation was used as one of the tools to explore the role of citizens in the use of health data. The results of the consultation show that people’s perspective on the secondary use of health data is embedded in their perception of health data. The trustworthiness of decision-making processes on the secondary use of health data was also identified as an important factor, especially when these decision-making processes include a plurality of views and actors. The relationship seems to depend strongly on what the health data will be used for and who will benefit from it.
Citizens also perceived health data as power. Although they tended to support the secondary use of health data, this was dependent on the conditions applied for using such data. They were looking for the right balance to maximise benefits while minimising the risks they perceived as possible when their data are used. To balance this power, they recommended that they should be given an opportunity to make meaningful and active choices, that data should be used in a way that makes it as difficult as possible to identify them, and that they expected more transparency about who accesses their data and for what purpose. They also trusted in the use of IT solutions and strong accountability mechanisms to protect their data.
The EHDS legislative proposal is an opportunity for policymakers to integrate public involvement into the secondary use of health data. These findings give a first indication of how this could be achieved in the implementation of the EHDS at European and national level.
TEHDAS has also explored the concept of health data altruism. The term data altruism refers to the voluntary sharing of data based on the consent of data subjects to the processing of their personal data, or the permission of data holders to allow the use of their non-personal data, for purposes of general interest without any reward beyond the compensation of costs. In this context, an analysis of the relevant literature was carried out, and a series of workshops were held with stakeholders to discuss data altruism as a way of sharing data and various aspects of citizen engagement.
The work also summarises lessons learned and recommendations for promoting data altruism mechanisms for the EHDS, including an analysis of data altruism organisations and business models, and the use of consent as the legal basis and citizen trust in data altruism.
The recommendations on data altruism addressed to European policymakers and data altruism organisations are intended to support the design and future implementation of the EHDS. This work can encourage policymakers and stakeholders from the ecosystem of the secondary use of health data to include the voice of citizens and patients in the definition and process of the secondary use of health data, to ensure its benefits are realised and its risks are minimised. Taking these steps will build public trust, which in turn will foster positive attitudes towards the secondary use of health data.
Sustainability requires dedicated efforts at both EU and Member State level
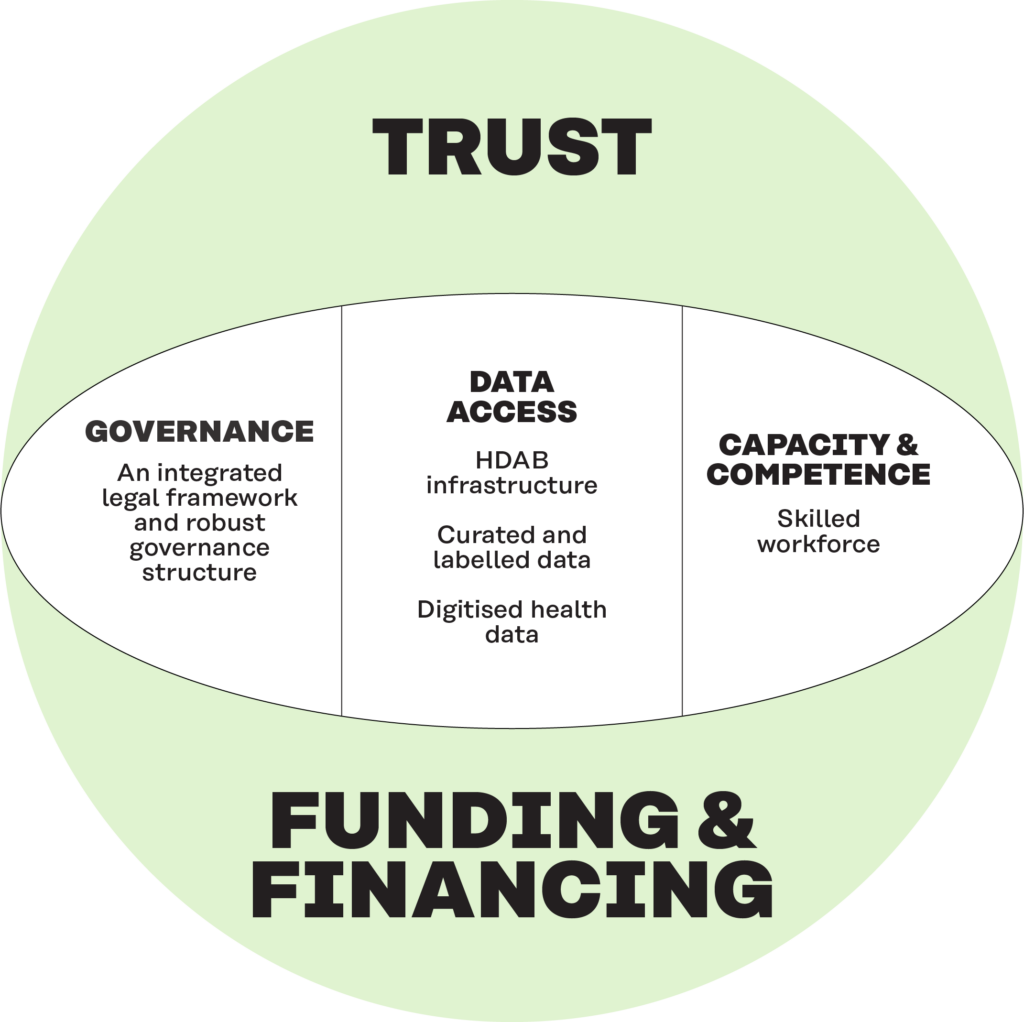
Sustainability has several dimensions in addition to funding and financing. These include a legal basis and governance, access to quality data, capacity and competence and trust.
Sustainability is an important aspect within the EHDS and the secondary use of health data. A sustainability framework was proposed, integrating five key dimensions: a clear legal basis and robust governance structure; access to quality data; capacity and competence; trust among citizens, professionals, and policymakers; and funding and financing.
The framework has been used to develop recommendations for action for Member States and the EU to ensure the sustainability of the EHDS through short, medium and long-term measures.
The conclusions of this work showed that EU funding plays a key role in achieving cross-border access to health information, but that Member States need to invest in their own national systems to make the information accessible.
The benefits, costs and resource requirements of data availability are different for different actors: data holders, bodies responsible for data access (Health Data Access Bodies, HDABs) and users. The largest share of costs seems to concern data acquisition, which is due to the costs of data preparation and extraction. Administrative fees and the costs of a secure operating environment are lower.
The framework served as a basis for developing recommendations for actions to be taken at the Member State and EU level to address the long-term sustainability needs of the EHDS. By considering these dimensions and recommendations, TEHDAS aimed to ensure the establishment of a sustainable and pragmatic EHDS that can effectively use health data for the benefit of citizens, researchers, and policymakers across Europe.
Figure 5. The five dimensions of sustainability
3. Towards a healthier Europe
The work towards better health and care is not yet complete, but thanks to the TEHDAS project, the cross-border use of health data in the EU has taken a major step forward. This work will continue, with a focus on the successful implementation of the EHDS, which will require broad collaboration across Europe.
Overall achievements
TEHDAS has developed concrete concepts and proposals to promote the secondary use of health data and the development of the EHDS. The results have been widely used by stakeholders and are reflected in the proposal for the EHDS. TEHDAS assessed the readiness for the EHDS in many Member States and increased the readiness for digital health, while contributing to the development of legislation in this area. Significant progress has been made in data quality and in ensuring semantic and technical interoperability to support the future implementation of the EHDS.
The joint action has been of strategic importance for Europe and therefore broad collaboration with key stakeholders has been a key element in all TEHDAS’ activities, enabling the integration and relevance of different perspectives and needs, and promoting dialogue with policymakers at national and European level. Importantly, the TEHDAS stakeholder forums and other events have brought together key stakeholders and provided, a platform for an exchange of knowledge.
A major effort has been made to translate the policy work into a broader societal impact by discussing and raising awareness of the legal proposal, while at the same time summarising the key activities and results stemming from the work of TEHDAS. This effort can be seen, for instance, in the vast number of presentations and speaking engagements made by the TEHDAS coordination team and TEHDAS’ partners throughout the project.
Furthermore, in order to ensure that the frameworks, recommendations and guidelines developed are taken up in decision-making and put to further use, TEHDAS has stressed the need for clear communication to reach the relevant target audiences.
Above all, TEHDAS has acted as a neutral facilitator, bringing together European countries to advance the EHDS agenda. Most importantly, the project has provided a fertile ground for ensuring the development of legislation that is relevant and fit-for-purpose in this area and the future implementation of the EHDS. It has promoted access to health data across borders to boost research, innovation and policymaking for better public health, ultimately enabling better health for all.
Benefits
The vision of TEHDAS was that in the future, European citizens, organisations, research and innovation, communities and companies will benefit from secure and seamless access to health data regardless of where it is stored. TEHDAS developed several key concepts, policy options and recommendations necessary for addressing the realisation of the EHDS, thus laying the foundation for an increased use of health data and promoting common rules and guidelines in Europe for the secondary use of health data.
The TEHDAS joint action fostered dialogue with policymakers and other key stakeholders at national and EU level, raising awareness of the profound changes that are anticipated in both the regulatory and operational environments for the secondary use of health data. This has increased the readiness of the Member States and the Commission to set up the EHDS. The integration of different points of view and expertise has contributed to a common understanding, common practices and recognition of the need for the further development of the EHDS. This only underlines the importance and timeliness of the TEHDAS activities and results in supporting the development of the EHDS.
The delivery of transparent guidelines, recommendations and proposals developed in the context of the TEHDAS joint action has paved the way for faster and easier access to health data for research organisations, policymakers, heath service developers and health professionals, consulting and management organisations, the health industry and the public.
It is envisaged that, following the adoption of the legal instruments on the secondary use of data, accompanied by adequate funding and support for the practical implementation of the EHDS, numerous benefits for the secondary use of data will start to emerge.
Through the implementation of EHDS and, through HDABs, researchers and innovators will eventually have access to high-quality data in a more efficient and cost-effective way, while ensuring the privacy of the patients and matters of security. Business will benefit from the single market for data, with new opportunities for innovative solutions and services on a level-playing field with the same technical standards and specifications. Furthermore, regulators and policymakers will have easier access to health data, enabling the data-driven and knowledge-driven steering of health systems and evidence-based policymaking to improve public health.
Health data can be used to advance knowledge of the causes of diseases and risk factors, and to better understand health conditions and treatments. There is a great potential to improve disease prevention and early warning mechanisms through significant advances in artificial intelligence and advanced data analytics and tools.
To take full advantage of these novel opportunities and create value for society as a whole, more attention should be paid to fostering an enabling environment and legal and operational clarity. Ensuring the high-quality of data and smooth access to it is crucial, while putting in place the necessary operational structures and infrastructures are also a key, bearing in mind the different end-users and benefits to them.
The way forward
The nature of the project was preparatory and its results have been widely used in other initiatives, such as the EHDS2 Pilot, and of course in the planning of the EHDS.
Legislative negotiations are ongoing in the European Parliament and in the Council of the European Union. The European Parliament is expected to hold the plenary vote on its position in the autumn of 2023 and the EU Council is expected to finalise its position by the end of 2023. Once both institutions are ready, the trilogue negotiations can start involving the European Parliament, EU Council and the European Commission. The final legal text is expected in 2024 and, once adopted, will enter into force after a transitional period.
Not all issues will be finalised in the regulation. Implementing acts will be agreed after the regulation is adopted. These are legally binding decisions, such as technical specifications, that facilitate the harmonised implementation of the regulation.
A new joint action is planned to support the European Commission and the Member States in developing these important acts, continuing the successful work of TEHDAS. It will bring together Member States’ experts on the secondary use of health data and support the readiness for the implementation of the EHDS. The TEHDAS2 project is expected to start in the of summer 2024. To ensure the success of this follow-up project we need a wide range of expertise across Europe.
Writers
Editors
Markus Kalliola, Sitra
Elina Drakvik, Sitra
Maria Nurmi, Sitra
Contributors
Linda Abboud, Sciensano Sciensano Belgian institute for health
László Benzce, National Healthcare Service Center (OKFÖ)
Enrique Bernal-Delgado, The Institute for Health Sciences in Aragon (IACS)
Petronille Bogaert, Sciensano
Sérgio Dinis, Shared Services for Ministry of Health, EPE (SPMS)
Juan González-García, IACS
Coen van Gool, National Institute for Public Health and the Environment (RIVM)
Mario Jendrossek, The French Health Data Hub (FR-HDH)
Irene Kesisoglou, Sciensano
Ramón Launa, IACS
Malou Munkholm, Central Denmark Region (RM)
Cátia Pinto, SPMS
Michel Silvestri, Swedish eHealth Agency (SEHA)
Carlos Telleria, IACS
The coordinating team at Sitra
Markus Kalliola
Elina Drakvik
Maria Nurmi
Tapani Piha
Henna Keränen
Marja Pirttivaara
Taru Ryske
Vappu Santalahti
Kirsi Suomalainen
Disclaimer
The content of this deliverable represents the views of the author(s) only and is his/her/their sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the Consumers, Health, Agriculture and Food Executive Agency or any other body of the European Union. The European Commission and the Agency do not accept any responsibility for use of its contents.
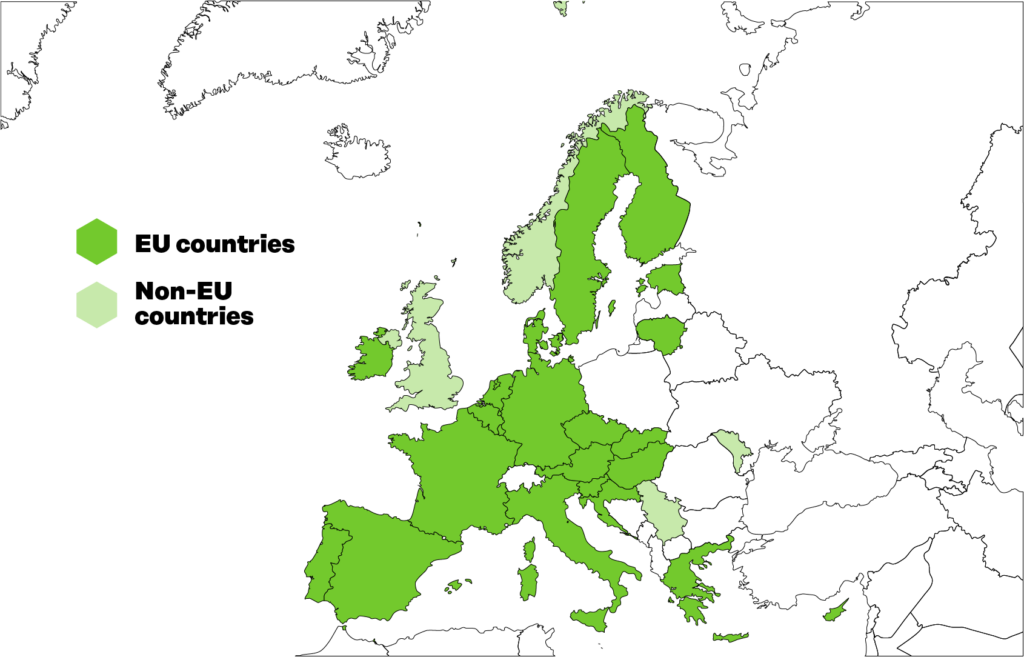
TEHDAS consortium
The TEHDAS Consortium includes main partners nominated by national governments and other organisations such as hospitals, research centres and universities.
Figure 1. 25 European countries participated in TEHDAS.
- Federal Ministry Republic of Austria Social Affairs, Health, Care and Consumer Protection, Austria
- Sciensano, Belgium
- Croatian Health Insurance Fund, Croatia
- Croatian Institute of Public Health, Croatia
- Ministry of Health of the Republic of Cyprus, Cyprus
- Ministry of Health of Czech Republic, Czech Republic
- Central Denmark Region, Denmark
- Central Denmark EU Office, Denmark
- Ministry of Social Affairs, Estonia
- The Finnish Innovation Fund Sitra, Finland
- CSC – IT Center for Science (CSC), Finland
- Finnish institute for health and welfare (THL), Finland
- Findata – Finnish Social and Health Data Permit Authority
- Technical Research Centre of Finland (VTT), Finland
- The French Health Data Hub, France
- National Institute of Health and Medical Research, France
- Hospices Civils de Lyon, France
- Université Toulouse III – Paul Sabatier, France
- Aix-Marseille University, France
- Agence du Numérique en Santé, France
- Federal Ministry of Health, Germany
- Research Data Centre of the Federal Statistical Office, Germany
- Gematik GmbH, Germany
- Regional 6th Health Administration of Peloponnese – Ionian Islands Epirus & Western Greece, Greece
- National Healthcare Service Center, Hungary
- Semmelweis University, Hungary
- Department of Health, Ireland
- Health Research Board, Ireland
- Ministry of Health of Italy, Italy
- Ministry of Health of the Republic of Lithuania, Lithuania
- Ministry for Health – Government of Malta, Directorate Health Information and Research, Malta
- Transplant Agency of the Republic of Moldova, Moldova
- Ministry of Health, Welfare and Sports, Directorate Information Policy, Netherlands
Nictiz, Netherlands - National Institute for Public Health and the Environment, Netherlands
- The Norwegian Ministry of Health and Care Services, Norway
- Norwegian Directorate of Health, Norway
- Norwegian Directorate for eHealth, Norway
- Norwegian institute for public health, Norway
- Shared Services of the Ministry of Health, EPE, Portugal
- Institute of Public Health of Republic of Serbia “Dr Milan Jovanovic Batut”, Serbia
- National Institute of Public Health, Slovenia
- The Institute for Health Sciences in Aragon, Spain
- Swedish eHealth Agency, Sweden
- Public health agency of Sweden, Sweden
- National Board of Health and Welfare, Sweden
- The NHS Confederation, United Kingdom
- Wellcome Sanger Institute, United Kingdom
In addition, selected advisors gave direct input to TEHDAS proposals throughout the project and a wide range of European stakeholders were involved.
Further reading
Links retrieved on 30 August 2023
TEHDAS joint action. The website of the joint action.
Sitra, the project co-ordinator: Joint action Towards the European Health Data Space.
European Commission. European Health Data Space.
European Commission’s Funding and Tender’s portal. Towards the European Health Data Space Joint Action.















